Introduction to T cells and B cells
T cell and B cell lymphocytes work together to recognize foreign substances called antigens. As the primary agents responsible for adaptive immunity, T cells and B cells are sometimes called the “special ops” of the immune system. Inherent structural features of the B and T cell receptors are what provide antigen binding specificity.
Lymphocytes originate from a lymphoid progenitor cell in a process called hematopoiesis. In hematopoiesis, stem cells within bone marrow differentiate into myeloid progenitors or lymphoid progenitors, which then specialize into about a dozen different cell types including blood cells, platelets, and macrophages—which are all myeloid in origin—and lymphocytes.
Lymphocytes can be further differentiated into B cells, T cells, and natural killer cells. While natural killer cells recognize general signals of immune stress such as inflammation, B and T cells recognize foreign antigens specifically via hyper-variable B cell and T cell receptors (BCRs and TCRs). B cells recognize free, unprocessed antigens. T cells recognize antigens within a complex of cell surface proteins called the major histocompatibility complex (MHC) on the surface of antigen-presenting cells (also called accessory cells).
The proper function of B cells and T cells is essential to protect the body from foreign invasion by viruses or bacteria. This proper function is contingent on T and B cell structure, as it dictates their activation and downstream function. When these systems go haywire, the body is left susceptible to diseases and cancer. In the case of auto-immune disease, the immune system itself can even become a detriment. By studying the unique composition of diverse B cells and T cells on both a macro (large population) and micro (individual) level, we can gain insights into how to treat or prevent diseases.
For case studies of BCR and TCR research, see our page on sequencing the immune repertoire.
Introduction to Adaptive Immunity
Our bodies protect us from foreign invasion through two main defense systems: innate immunity and adaptive immunity. Innate immunity is a general defense mechanism that works non-selectively to keep out potential threats. Physical barriers like skin and chemical responses like inflammation both constitute innate immunity. The defining feature of innate immunity is that the response is more or less the same regardless of invasion type.
Adaptive immunity, on the other hand, is a specific, acquired response to particular invaders. In adaptive immunity, toxins or foreign substances, called antigens, are recognized specifically via molecular signatures. The full breadth of threats that our adaptive immune system can recognize changes overtime as we are exposed to new antigens.
Adaptive immunity depends on the diversity of B cell and T cell receptors. The individual components of BCRs and TCRs achieve diversity through random recombination of the genes that encode them. In BCRs, this diversity is further expanded via somatic hypermutation.
For more information, see our page on diversity and differentiation in the adaptive immune system.
When a BCR or TCR recognizes a foreign antigen, the cells housing that receptor proliferate in a process called clonal expansion. Most of these newly made cells will die off after the antigen is destroyed, but some of them are destined to live on as memory B or T cells. This new population of memory cells allows for a faster response when the same antigen is encountered again. Vaccines work by priming the adaptive immune system to respond to a particular pathogen by introducing antigens in the absence of the disease. The lymphocytes that recognize those antigens proliferate and create memory cells, so that if the body is challenged by the actual disease in the future, the adaptive immune system is ready to respond quickly.
T cell structure and function
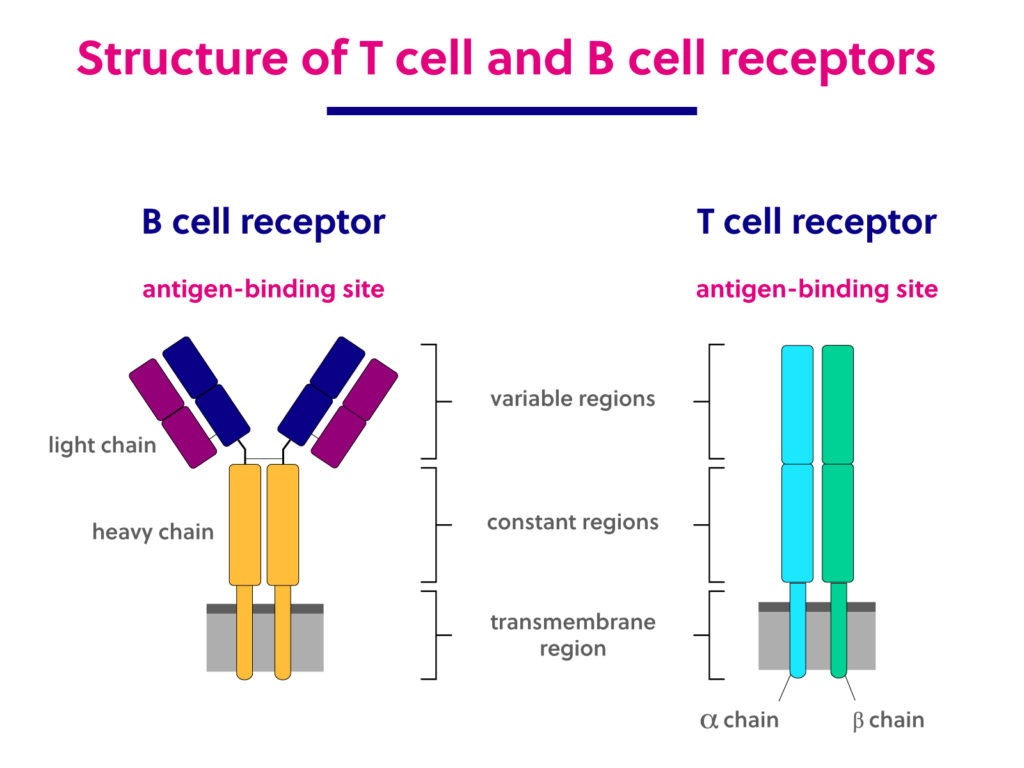
T cell receptors are made up of two polypeptide chains that together compose one antigen binding region. Approximately 95% of TCRs are composed of an alpha and a beta chain, while the remaining 5% of TCRs are made up of gamma and delta chains. The T cell receptor structure is maintained by a disulfide bond linking the two chains together. Complementary determining regions (CDRs) are key structural features that lie within the variable region and provide the specificity in antigen binding.
There are multiple types of T cells, and each has a specialized function. Cytotoxic T cells, also known as Killer T cells, generally target cancer, virally infected, or damaged cells. Killer T cells respond to antigens by releasing cytotoxic granules that lead to apoptosis. Helper T cells help recruit B cells and other cells involved in the immune response by releasing cytokines. Memory T cells have an extended lifetime and help to recognize antigens to which they were previously exposed.
B cell structure and function
B cell receptors are made up of four peptides – two light chains and two heavy chains – that comprise two antigen-binding regions. Light chains are classified as either kappa or lambda, while the heavy chains can be IgG, IgA, IgM, IgD, or IgE isotypes.
B cells can be activated in two ways: T cell-dependent activation or T cell-independent activation. During T cell-dependent activation, B cells absorb the antigen and then present pieces of the antigen on their surface via a major histocompatibility complex (MHC). Helper T cells can then recognize those antigens via the MHC and activate the B cells. For T cell-independent activation to take place, the B cell must both encounter an antigen and receive a “danger signal,” which is a signal that an attack is occurring.
Activated B cells can then either become effector B cells or memory B cells. Effector B cells, also called plasma cells, produce antibodies. Antibodies work as tags or alarms to target invading agents for destruction by other immune agents like macrophages. Memory B cells, like memory T cells, help the immune system respond more quickly to future invasions by the same agent.
Lymphocyte-related diseases and treatment
When something goes wrong with the adaptive immune system, disease can result. Auto-immune diseases occur when B and T cells falsely recognize molecules that are not foreign as a threat. Lymphocytes are also involved in allergic reaction responses. In HIV infection, B cells and T cells become exhausted and no longer function properly. Finally, like any cell, B and T cells can mutate and divide uncontrollably, resulting in lymphoma. An improved understanding of the lymphocyte landscape within populations or individuals can help lead to improved tests and treatments for these diseases.
T cell and B cell associated therapies are an important component of cancer treatment. For example, some cancer cells produce molecules that can deactivate T cells. These cancer cells hijack the natural systems that evolved to turn T cells off when an infection has cleared. Checkpoint inhibiting drugs prevent this mechanism so that T cells will not be prematurely inhibited by cancer cells.
An exploratory but promising type of treatment called CAR-T cell therapy uses T cells to fight B cell lymphomas. In CAR-T therapy, a patient’s T cells are extracted, modified so that they recognize B cell surface proteins as antigens, and reintroduced. The modified T cells destroy cancerous as well as healthy B cells. Before long, the modified T cells are circulated out of the body, and the supply of normal lymphocytes is regenerated.
For more examples relating TCR and BCR research to medical applications, see our page on the immune repertoire and adaptome.